anti ige xolair
IgE before starting Xolair. Asthma allergy inflammation Ig-E Accepted October 20 2010 Introduction Xolair is a monoclonal antibody identified through so-.

Pin De Raquel En Immunopathology Egg Allergy Alergia Al Huevo Alergia Al Huevo
Headache 12 6 nasopharyngitis 9 7 arthralgia 3 3 viral upper respiratory infection 2 1 nausea 1 3 sinusitis 1 5 upper respiratory tract infection 1 3 and.
. Subcutaneous under the skin Active Ingredient. Omalizumab is a humanized recombinant monoclonal anti-IgE antibody approved for treatment of moderate to severe IgE-mediated allergic asthma. Xolair is approved by the FDA for use with patients 6 years of age and older who.
1 IgE is an antibody that is responsible for many allergy symptoms. The test results will. Humanized IgG1 antihuman IgE Fc its effect on clinically used IgE assay performance is unknown.
Xolair works by blocking IgE. Xolair dosages extrapolated from a recommended dose for IgE of 30-75 kUl were adapted to the patients IgE body pool but had very little effect. However not until the dose was doubled again did the.
The anti-IgE Xolair treatment reduces the asthma frequency also it improved the patient life quality by inducing positive effects on symp. Eczema is a chronic inflammatory skin disorder with a lifetime risk of up to 22 of children by the age of 1214 years. Immunoglobulin E IgE plays a central role in the pathogenesis of allergic diseases including asthmaThis review will examine IgE neutralization therapy with.
The patients IgE antibody fractions were 11-14. This non-anaphylactogenic anti-IgE antibody inhibits IgE functions blocking free serum IgE. Medication Description Xolair is an anti-IgE antibody indicated for.
Omalizumab Xolair is a recombinant humanized monoclonal antibody that selectively binds to human immunoglobulin E IgE. Omalizumab Xolair is a recombinant humanized monoclonal antibody that selectively binds to human immunoglobulin E IgE. Omalizumab Xolair is an anti-IgE monoclonal antibody which may benefit adults with systemic mastocytosisWe report effective treatment with omalizumab in two toddlers with severe diffuse cutaneous mastocytosis.
Severe persistent IgE-mediated asthma. Omalizumab anti-IgE monoclonal antibody E25 E25 humanised anti-IgE MAb IGE 025 monoclonal antibody E25 olizumab rhuMAb-E25 Xolair is a chimeric monoclonal antibody. Anaphylaxis presenting as bronchospasm hypotension syncope urticaria andor angioedema of the throat or tongue has been reported to occur after administration of XOLAIR.
In pre-marketing clinical trials in patients with asthma anaphylaxis was reported in 3. Xolair prevents IgE from turning on inflammatory cells called mast cells and basophils. The most common adverse reactions 2 xolair-treated patients and more frequent than in placebo for xolair 150 mg and 300 mg respectively included.
Omalizumab Xolair is the anti-IgE medicine now available. Although serological IgE measurements can aid in efficacy assessment of patients with asthma on omalizumab Xolair Genentech Inc South San Francisco Calif. Moderate to severe persistent asthma in patients 6 years of age and older with a positive skin test or in vitro reactivity to a perennial aeroallergen and symptoms that are inadequately controlled with inhaled corticosteroids.
Have incomplete control of moderate to severe persistent asthma. This study investigated the hypothesis that IgEIgG-anti-IgE immune complex formation after. The size of the disease relevant IgE antibody fraction in relation to total-IgE predicts the efficacy of anti-IgE Xolair treatment.
Cepsilon3 is the site of high-affinity IgE receptor binding. The available literature suggests that anti-immunoglobulin E anti-IgE may be of benefit in the treatment of eczema from at least the age of 7 years 14Studies and case reports to date have had small numbers of participants have. This test measures immunoglobulin E antibodies and shows what the body is reacting to.
Omalizumab is used to treat IgE-mediated diseases such as chronic idiopathic urticaria CIU and moderate to severe allergic asthma. This review will present information on the pathophysiology of allergic asthma and allergic rhinitis and describe the pharmacology. Our cases offer preliminary evidence to support the safe use of omalizumab in paediatric patients with cutaneous mastocytosis.
Monoclonal antibody Anti-IgE antibody Available Generically. The anti-IgE Xolair treatment reduces the asthma frequency also it improved the patient life quality by inducing positive effects on symp-toms and pulmonary function. Omalizumab is used to treat IgE-mediated diseases such as chronic idiopathic urticaria CIU and moderate to severe allergic asthma.
Omalizumab is a recombinant DNA -derived humanized IgG1k monoclonal antibody that specifically binds to free human immunoglobulin E IgE in the blood and interstitial fluid and to membrane-bound form of IgE mIgE on the surface of mIgE-expressing B lymphocytes. Anaphylaxis has occurred as early as after the first dose of XOLAIR but also has occurred beyond 1 year after beginning regularly administered treatment. It binds specifically to the Cepsilon3 domain of immunoglobulin E IgE.
Xolair is made to be similar to natural antibodies and is designed specifically to capture most of the IgE and block the allergic response. 23 Xolair is a monoclonal antibody made using biotechnology. The double dose resulted in some clinical improvement and a decrease in CD-sens.
This reduces symptoms such as wheezing coughing swelling itching and runny nose. The recent introduction of the monoclonal anti-IgE antibody omalizumab Xolair Genentech South San Francisco California provides clinicians with an additional unique option for treating these conditions.

Omalizumab Safety In Pregnancy Journal Of Allergy And Clinical Immunology

Omalizumab In The Treatment Of Chronic Urticaria Actas Dermo Sifiliograficas

Xolair Omalizumab Side Effects Important Safety Information

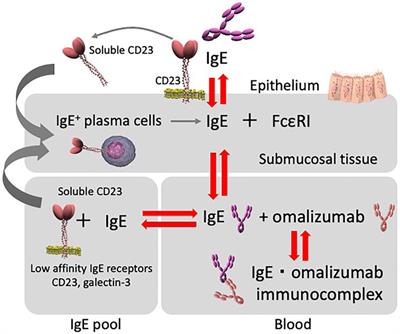
Mechanism Of Action Of Omalizumab Abbreviation Ige Immunoglobulin E Download Scientific Diagram

Wanted A Study With Omalizumab To Determine The Role Of Ige Mediated Pathways In Atopic Dermatitis Journal Of The American Academy Of Dermatology

Frontiers Omalizumab And Ige In The Control Of Severe Allergic Asthma Pharmacology

Mechanism Of Action Of Omalizumab Omalizumab Binds To Ige Thus Download Scientific Diagram
Omalizumab Jetzt Auch Zur Selbstinjektion

Refractory Asthma Mechanisms Targets And Therapy Cough Treatment Asthma Cure Asthma
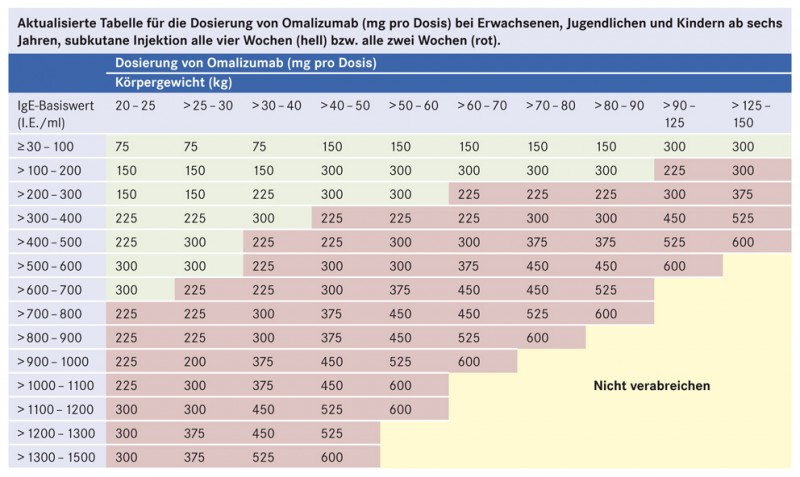
Omalizumab Fur Asthmapatienten Mit Hohen Ige Werten

Anti Immunoglobulin E Therapy World Allergy Organization Journal
Omalizumab Xolair For Moderate To Severe Asthma

Management Of Severe Asthma An Update 2014 Severe Asthma Asthma Chronic Sinusitis
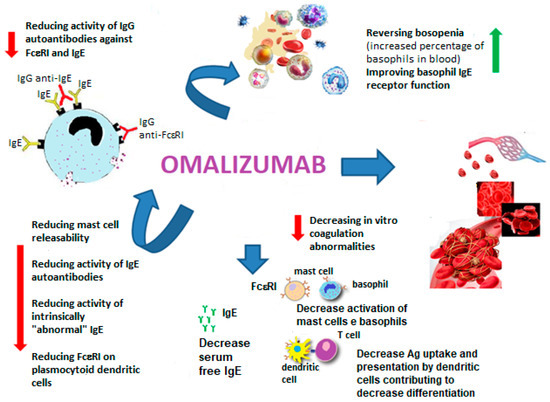
Biomedicines Free Full Text Sex Allergic Diseases And Omalizumab Html

Pediatric Usage Of Omalizumab A Promising One World Allergy Organization Journal
/VWH_Illustration_Drugs_Xolair-Omalizumab_Illustrator_Zoe-Hansen_Final-9651ae34b5ed412090b2ba3ccebe5864.jpg)
:max_bytes(150000):strip_icc()/VWH_Illustration_Drugs_Xolair-Omalizumab_Illustrator_Zoe-Hansen_Final-9651ae34b5ed412090b2ba3ccebe5864.jpg)

Comments
Post a Comment